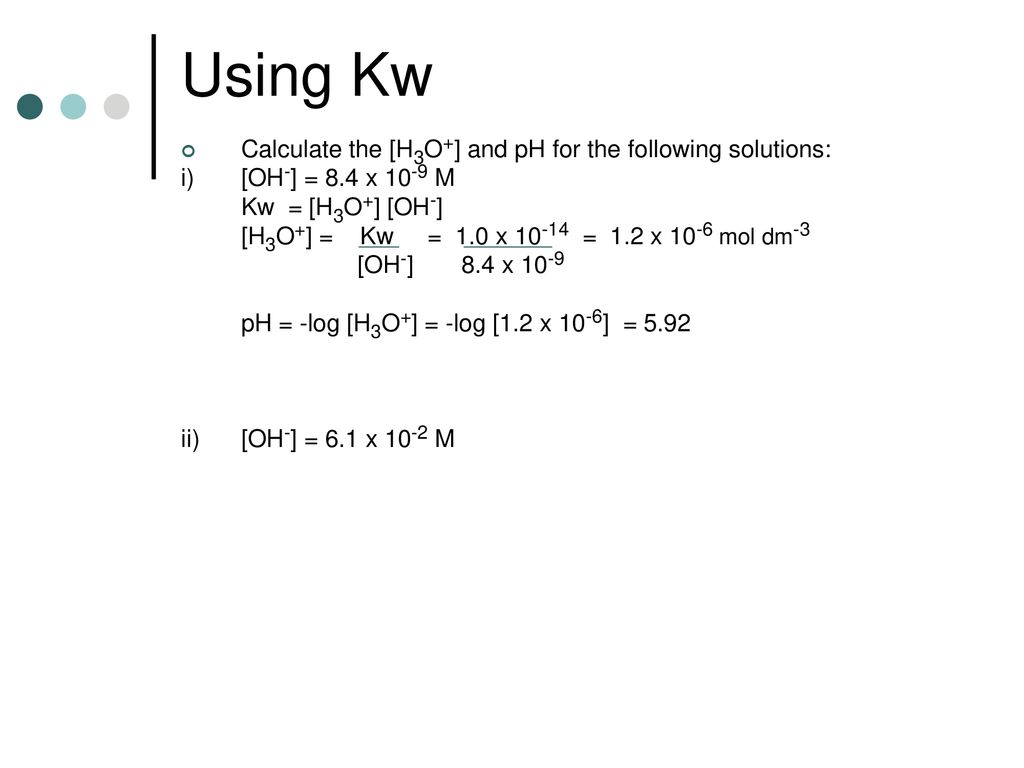
![PPT - Definition pH and pOH. Given pH, pOH, [H 3 O + ] or [OH¯], calculate the remaining values. PowerPoint Presentation - ID:5054819 PPT - Definition pH and pOH. Given pH, pOH, [H 3 O + ] or [OH¯], calculate the remaining values. PowerPoint Presentation - ID:5054819](https://image2.slideserve.com/5054819/slide13-l.jpg)
PPT - Definition pH and pOH. Given pH, pOH, [H 3 O + ] or [OH¯], calculate the remaining values. PowerPoint Presentation - ID:5054819
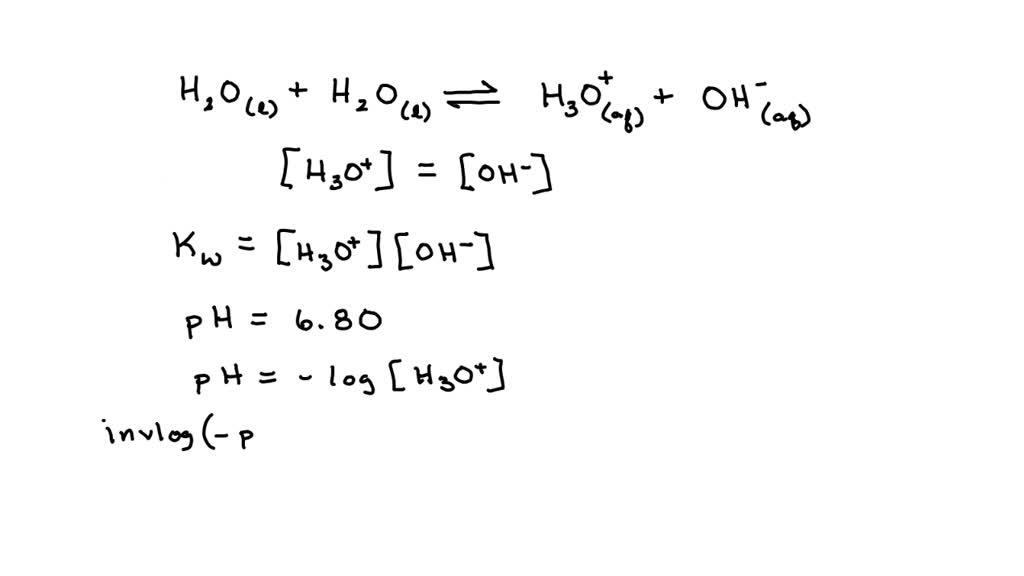
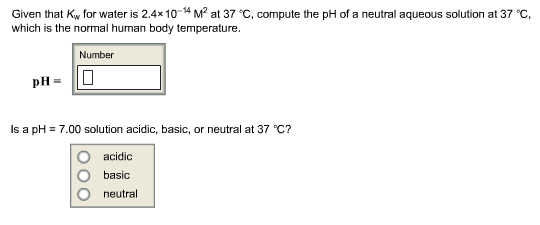
SOLVED: If the pH of pure water is 6.80 at 37 C, determine the value of KW at this temperature (it would be more relevant in medical applications that the value at 25 C).
The ionization constant for water (Kw) is 9.311 × 10−14 at 60 °C. What is the [H3O+], [OH−], pH, and pOH for pure water at 60 °C? Thanks. - TopScience - Quora
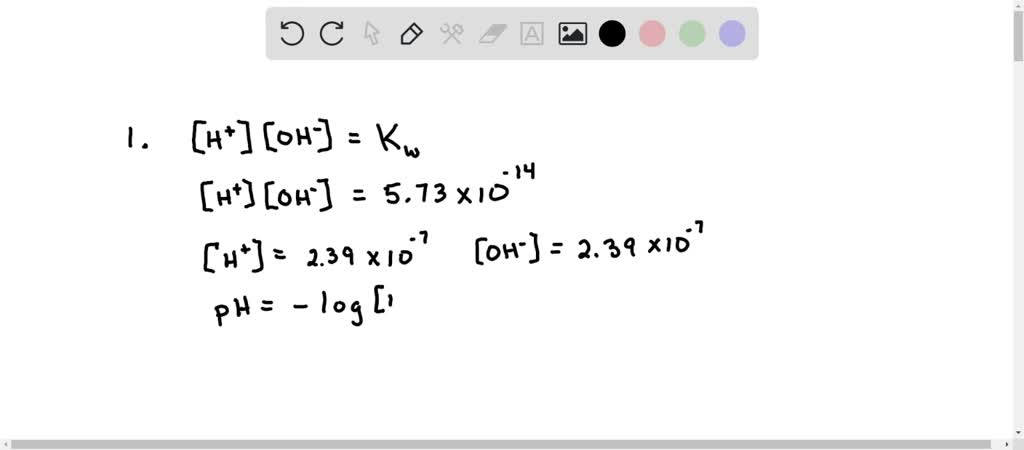
SOLVED: At 323 K, the value for Kw changes and is found to be 5.73 x 10 -14 . (i) Calculate the pH of water at this new, higher temperature. (1) (ii)
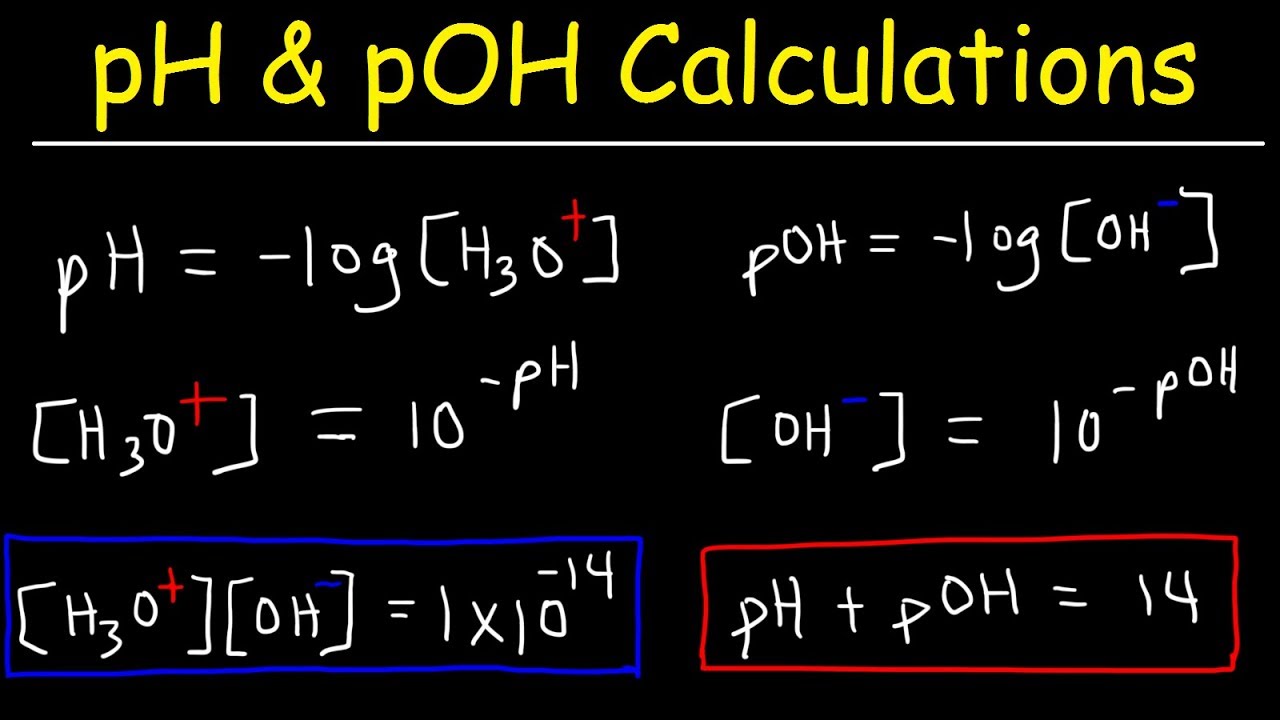
pH, pOH, H3O+, OH-, Kw, Ka, Kb, pKa, and pKb Basic Calculations -Acids and Bases Chemistry Problems - YouTube
![SOLVED: At 50°C, the value of Kw is 5.47 x 10^-14. a) Calculate the [H+] and [OH-] in pure water at 50°C. [H+] M [OH-] M b) What is the pH of SOLVED: At 50°C, the value of Kw is 5.47 x 10^-14. a) Calculate the [H+] and [OH-] in pure water at 50°C. [H+] M [OH-] M b) What is the pH of](https://cdn.numerade.com/ask_previews/ed96ab60-48fb-4213-8fd0-3c129172f46d_large.jpg)
SOLVED: At 50°C, the value of Kw is 5.47 x 10^-14. a) Calculate the [H+] and [OH-] in pure water at 50°C. [H+] M [OH-] M b) What is the pH of

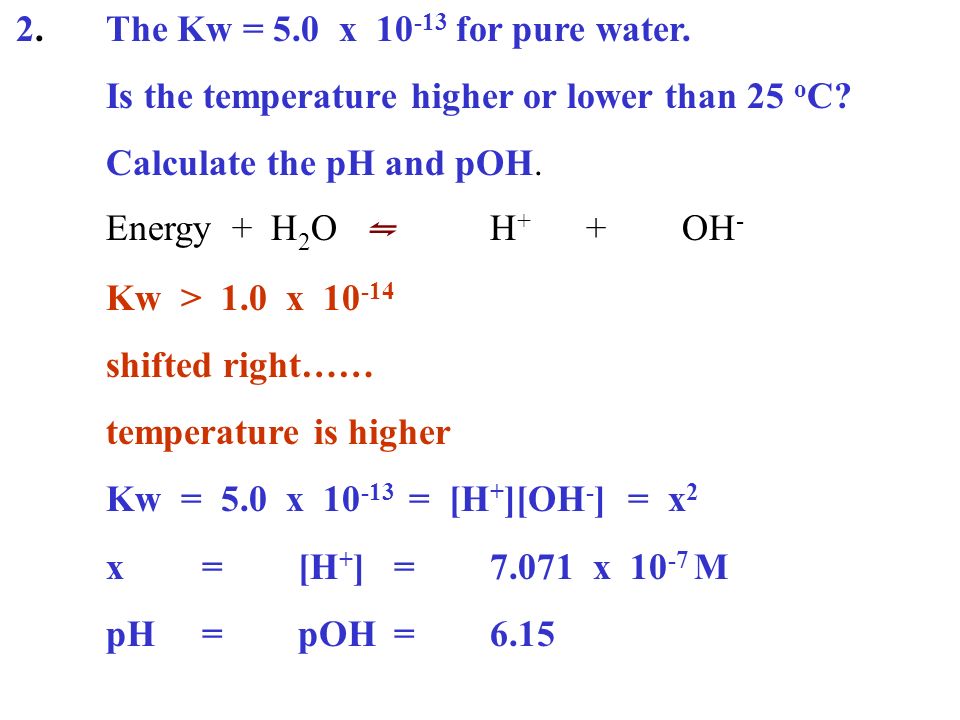

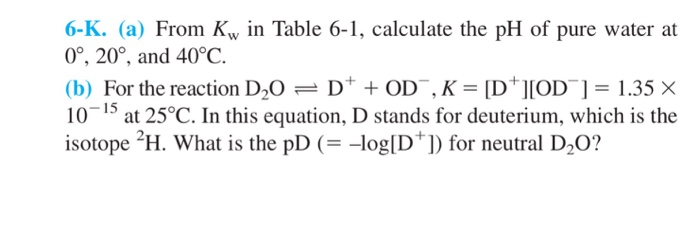
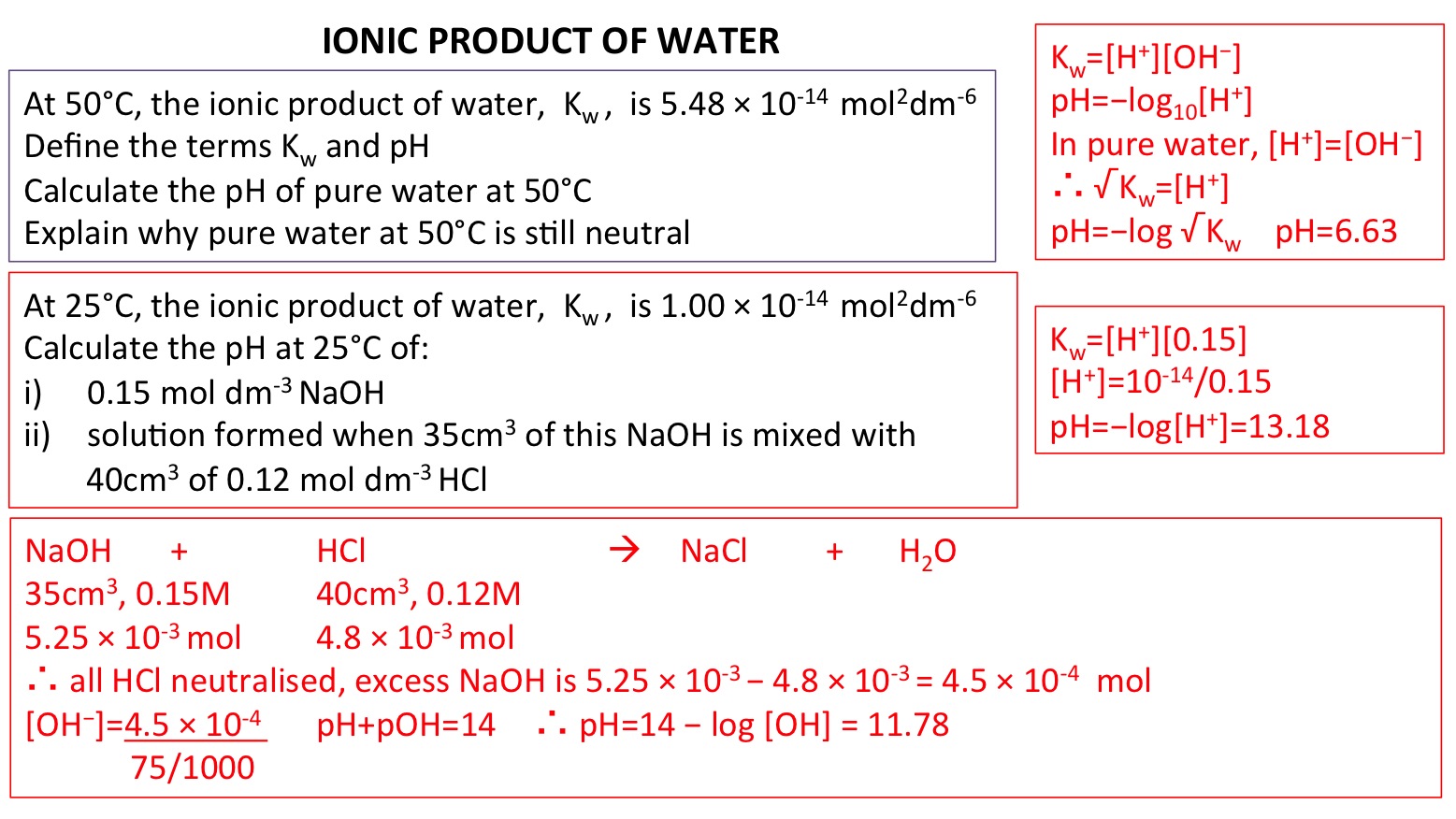
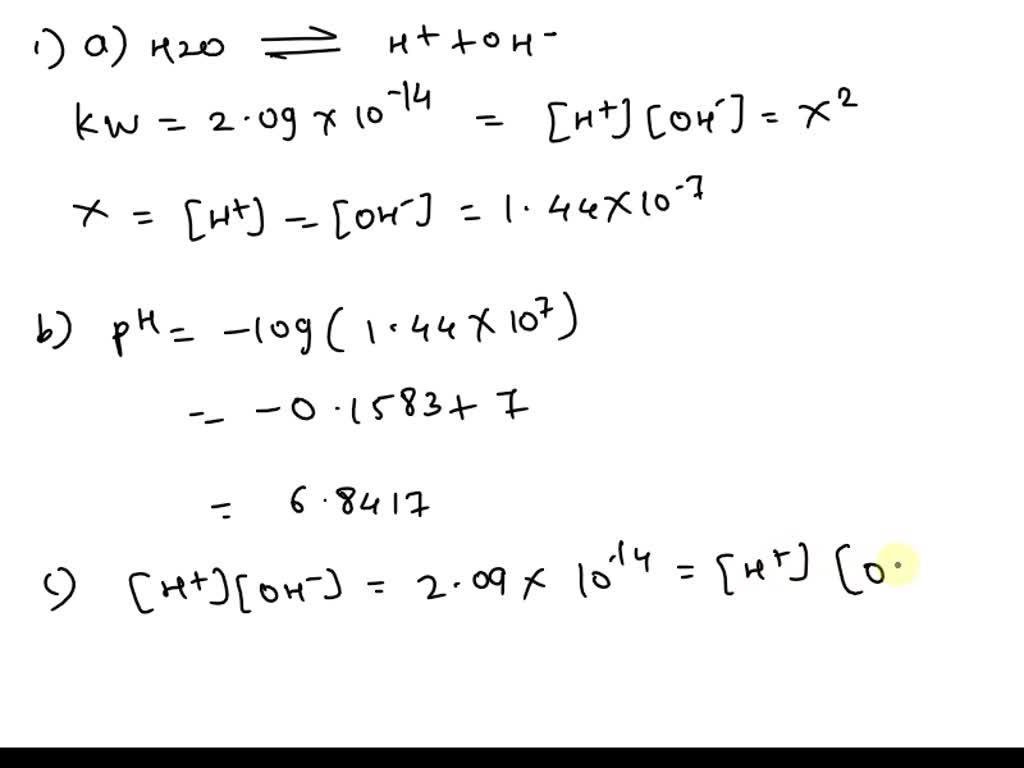

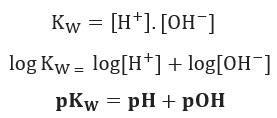
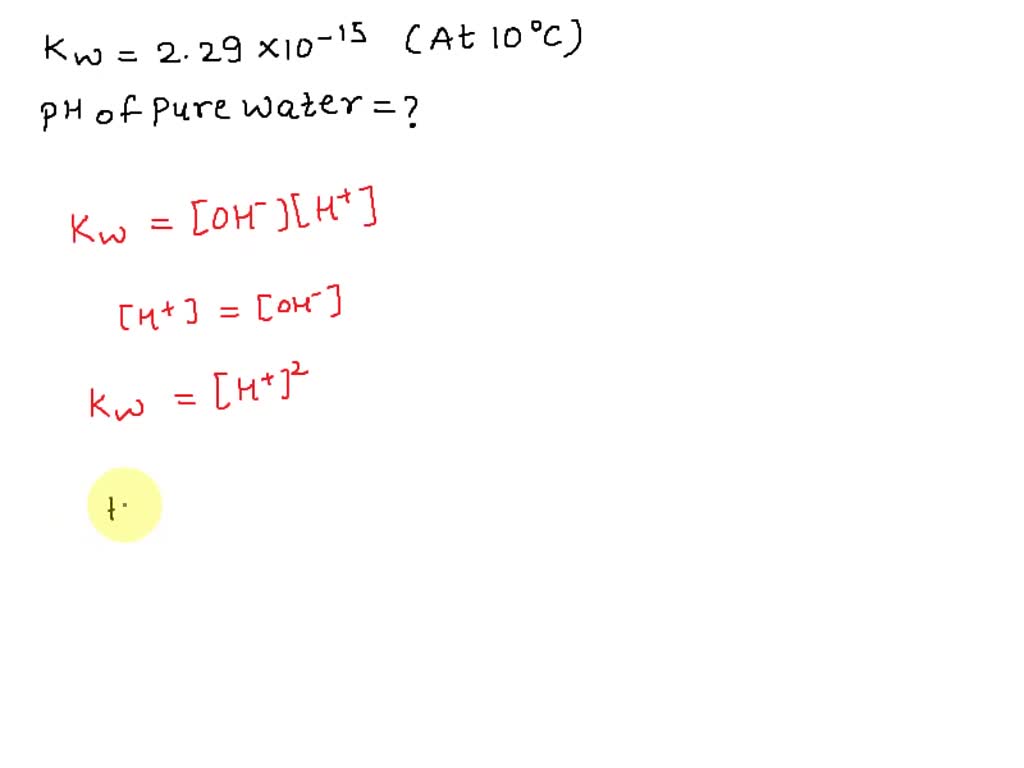

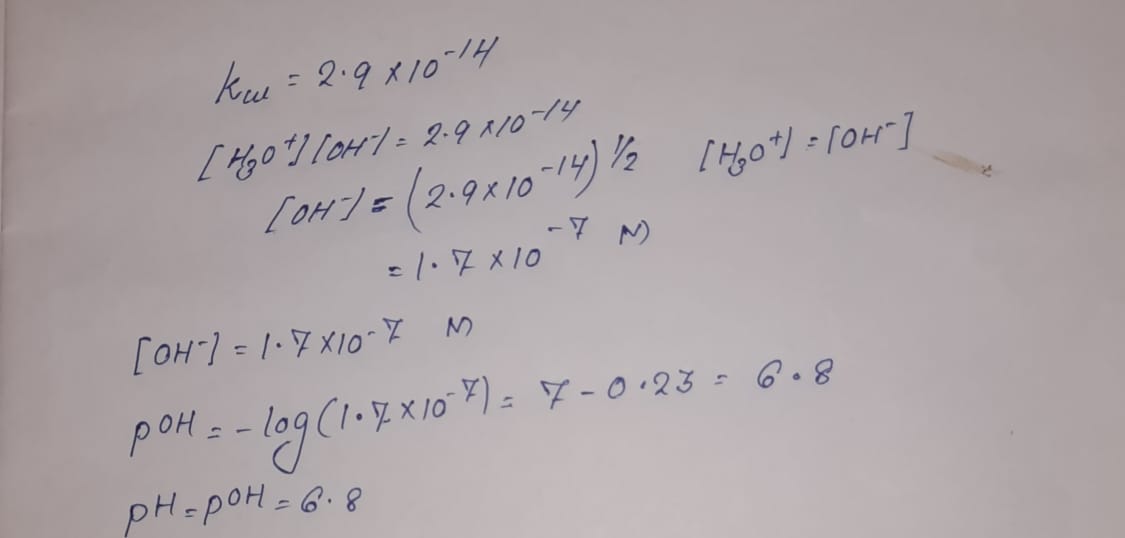
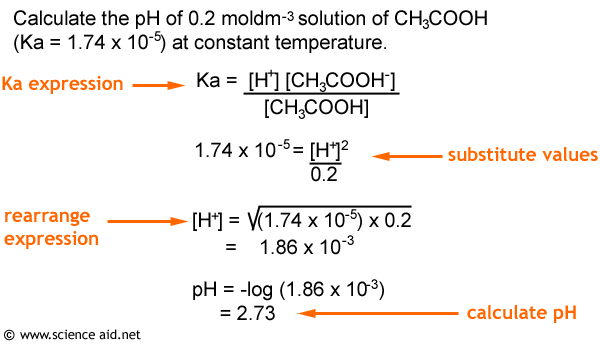
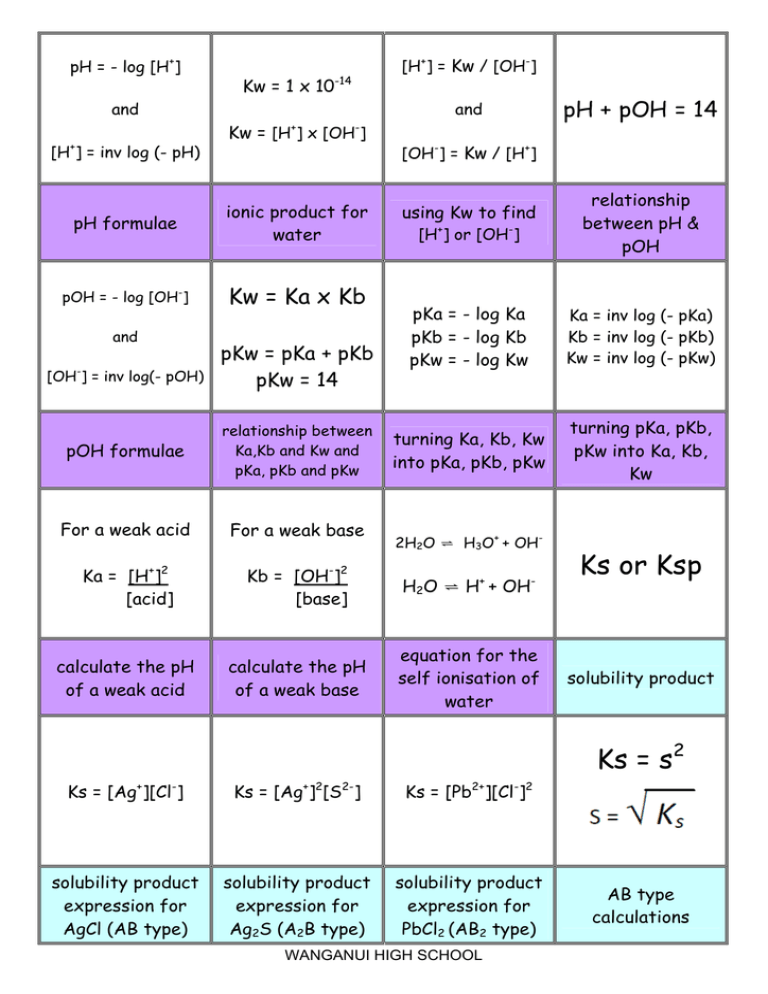
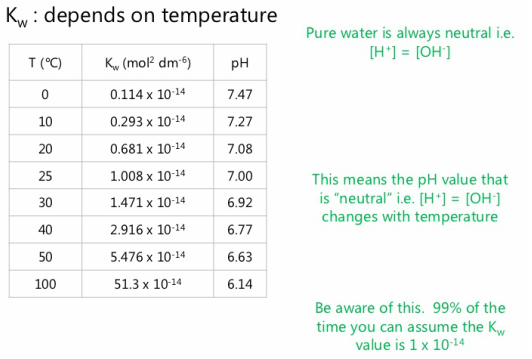
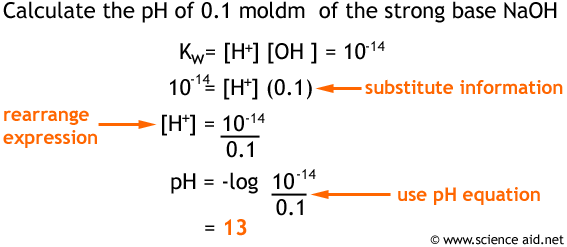
![Acids and Bases Part 4: Kw and Calculation of [H+] and [OH-] - YouTube Acids and Bases Part 4: Kw and Calculation of [H+] and [OH-] - YouTube](https://i.ytimg.com/vi/IvP_PxetNUw/maxresdefault.jpg)