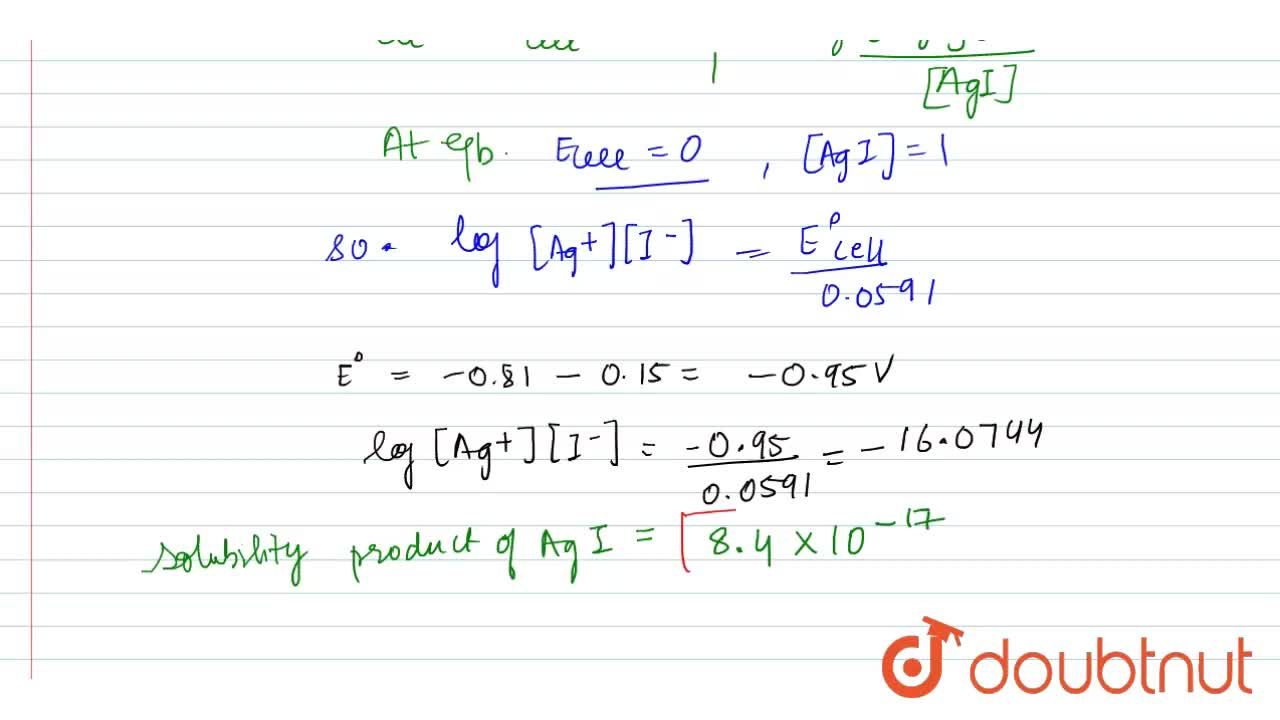
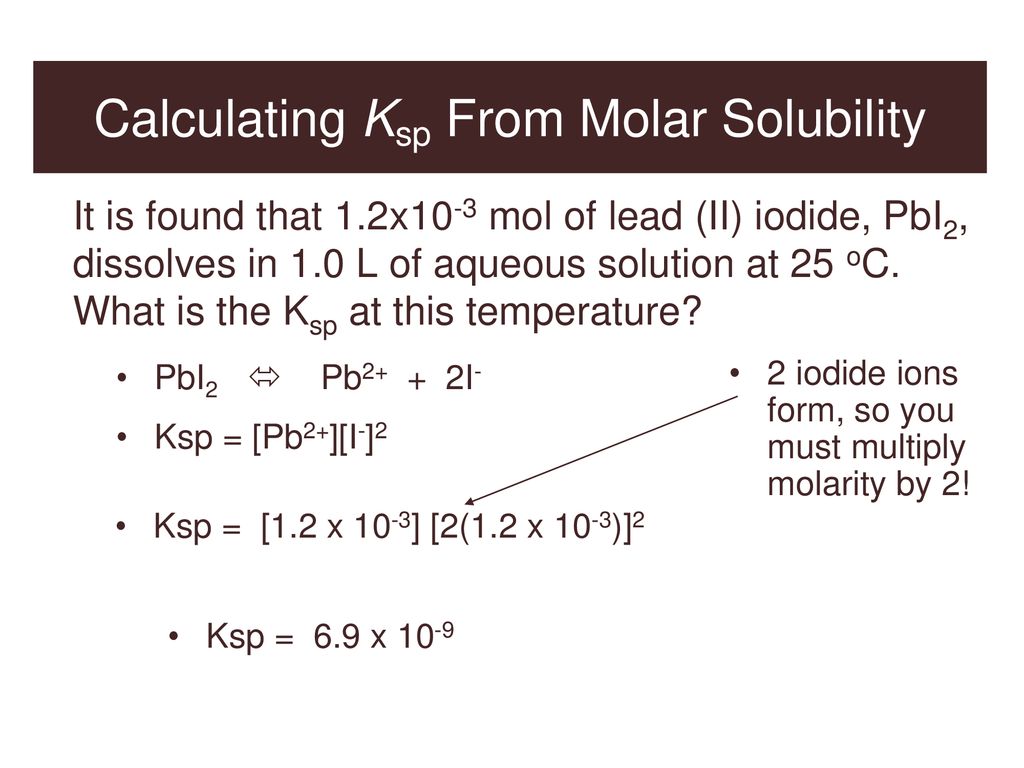
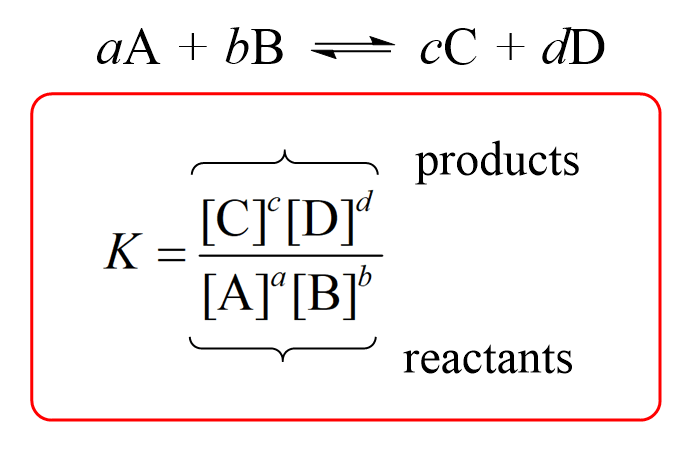
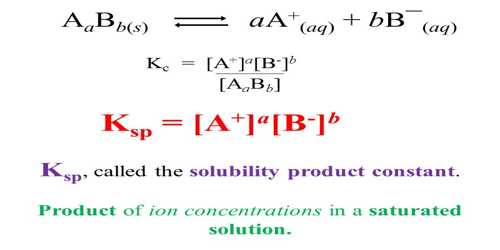
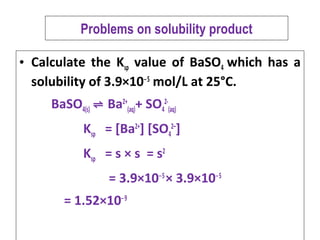
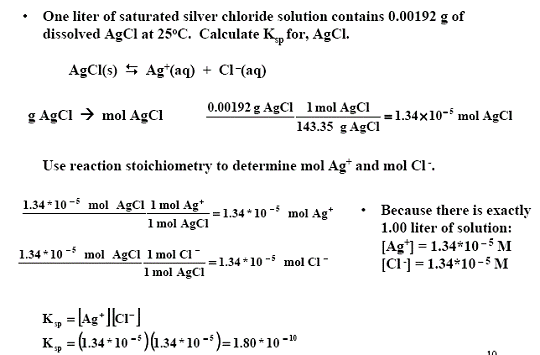
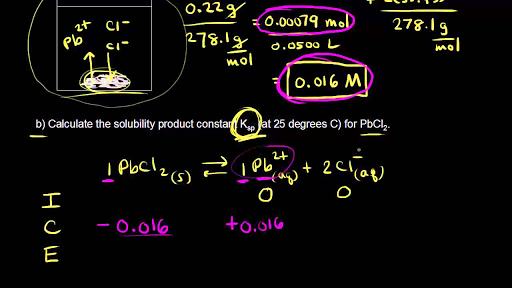
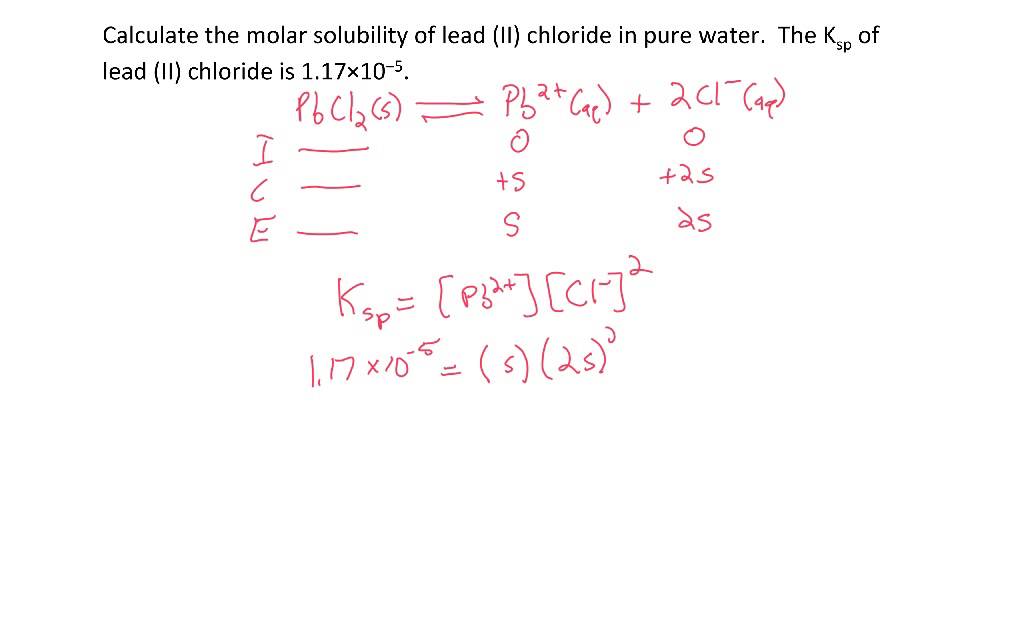
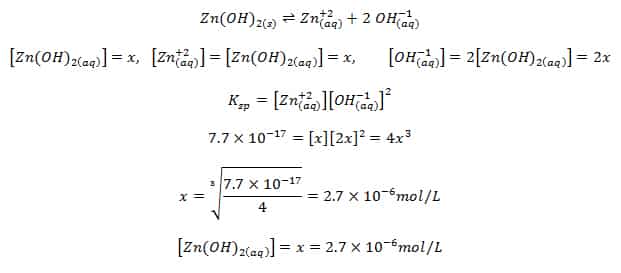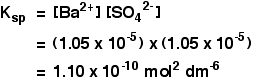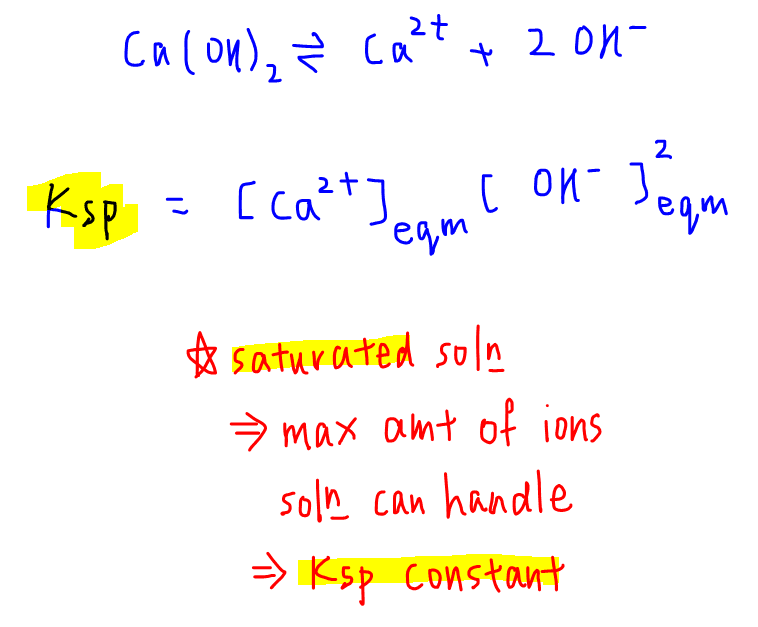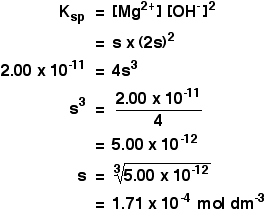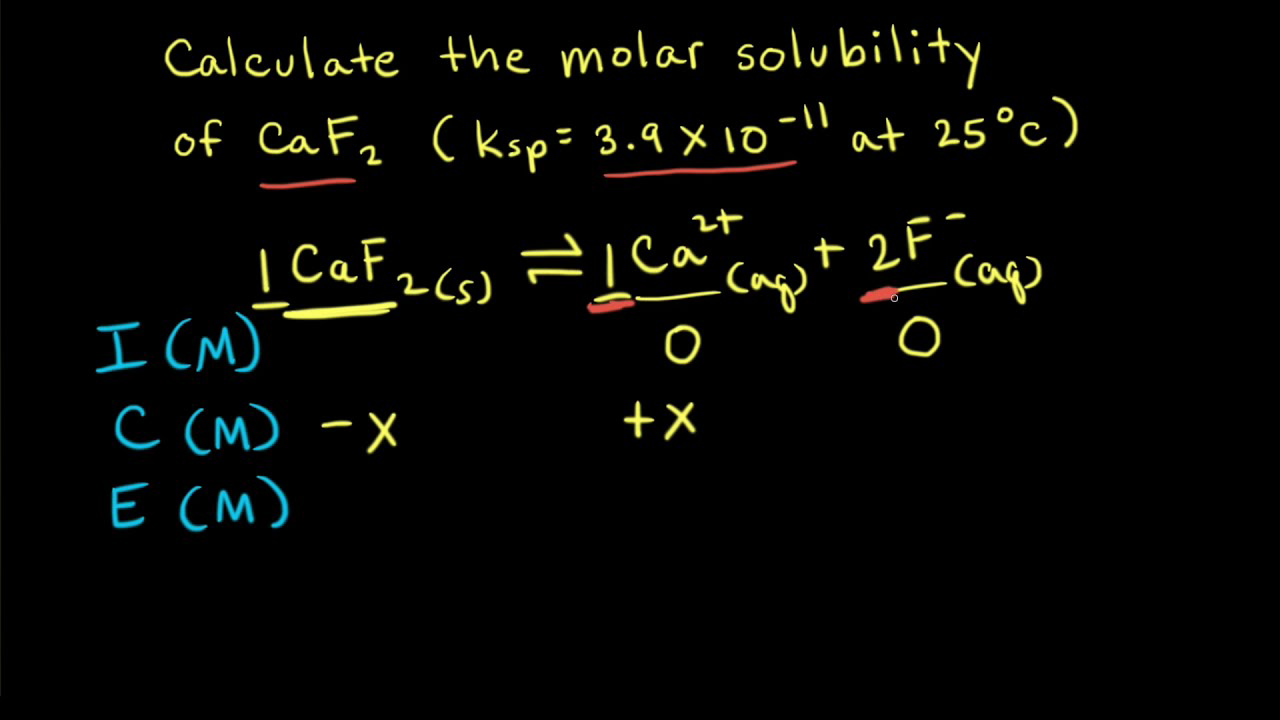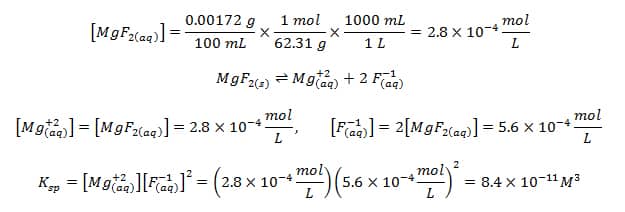The solubility product of Al(OH)3 is 2.7 × 10^-11 . Calculate its solubility in gL^-1 and also find out pH of this solution. (Atomic mass of Al = 27u ).
![The solubility product of PbCl2 at 298 K is 1.7 × 10^-5 . Calculate the solubility of PbCl2 in g/lit at 298K .Atomic weights : [ Pb = 207 and Cl = 35.5 ] The solubility product of PbCl2 at 298 K is 1.7 × 10^-5 . Calculate the solubility of PbCl2 in g/lit at 298K .Atomic weights : [ Pb = 207 and Cl = 35.5 ]](https://d1hhj0t1vdqi7c.cloudfront.net/v1/SFkyRXpPWTdqMnc=/sd/)
The solubility product of PbCl2 at 298 K is 1.7 × 10^-5 . Calculate the solubility of PbCl2 in g/lit at 298K .Atomic weights : [ Pb = 207 and Cl = 35.5 ]