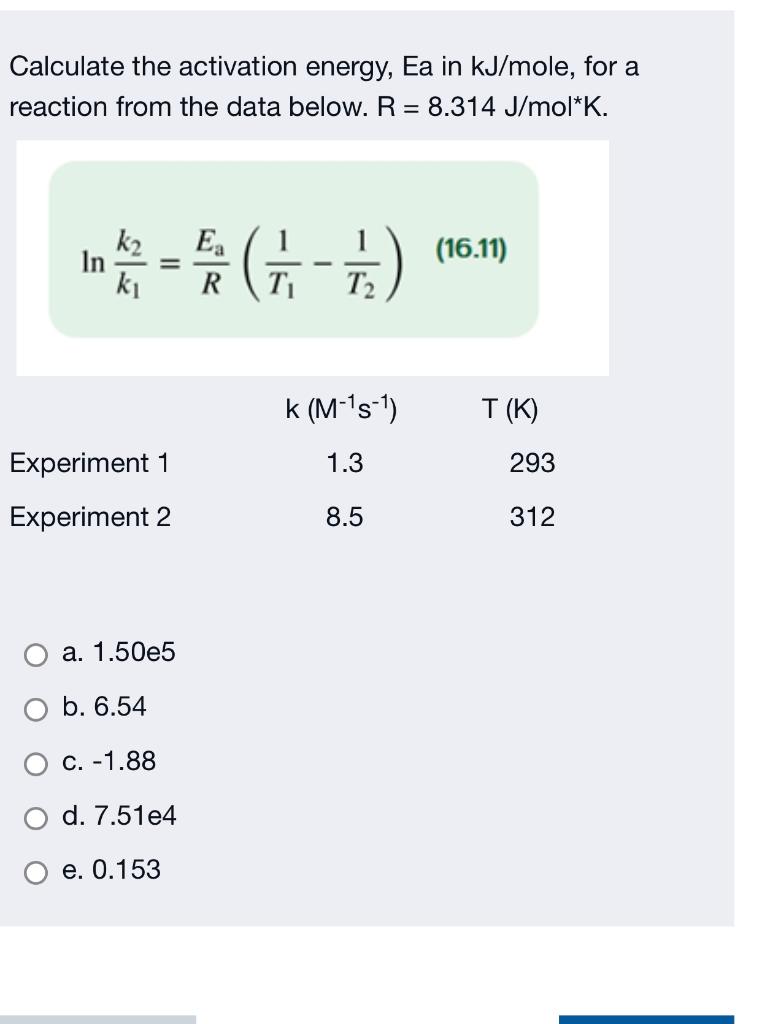
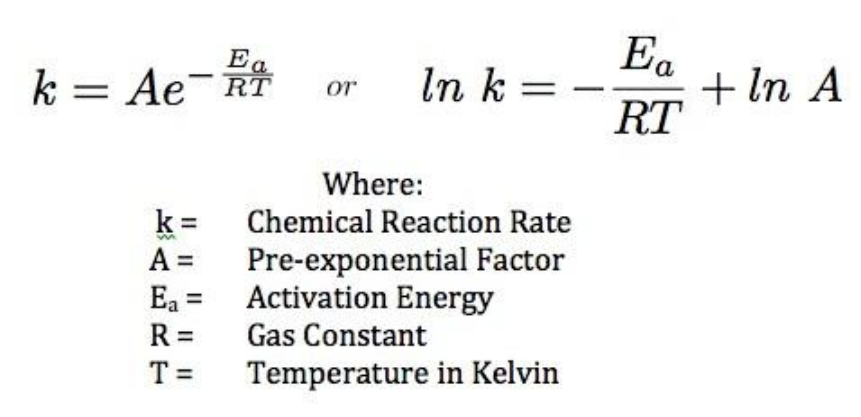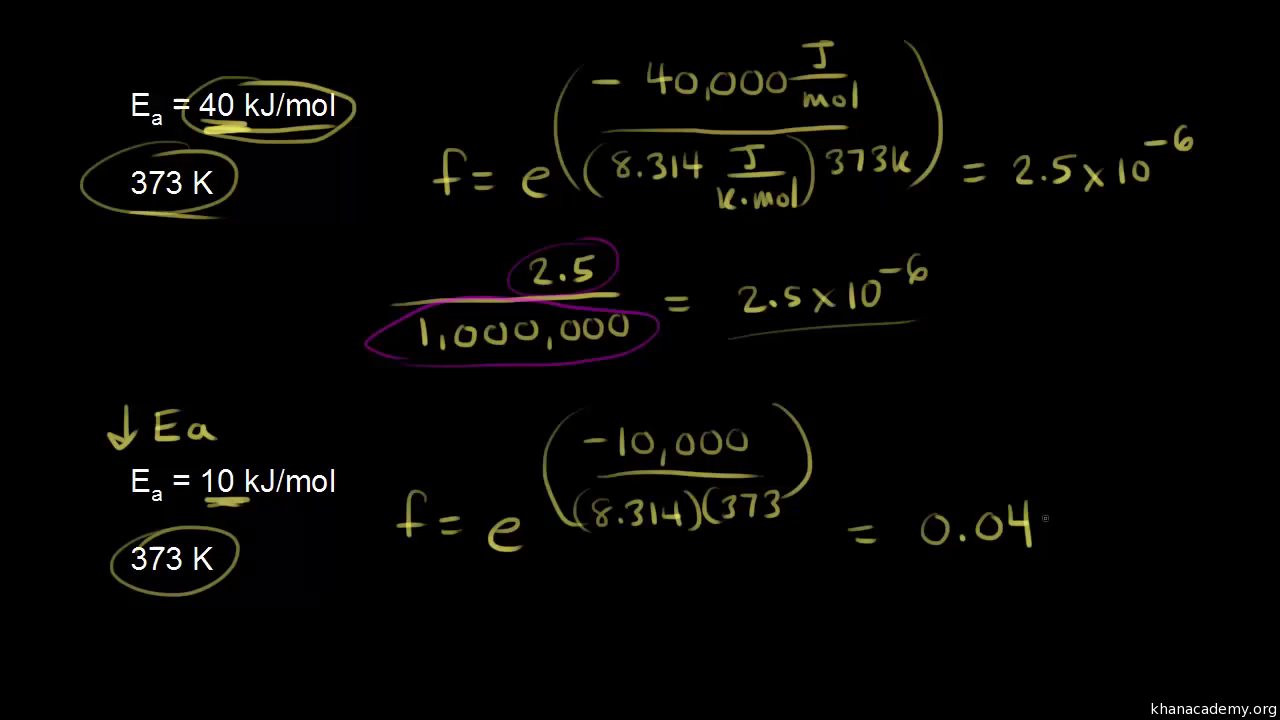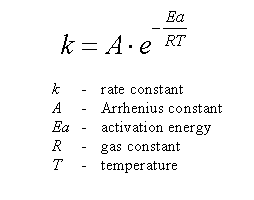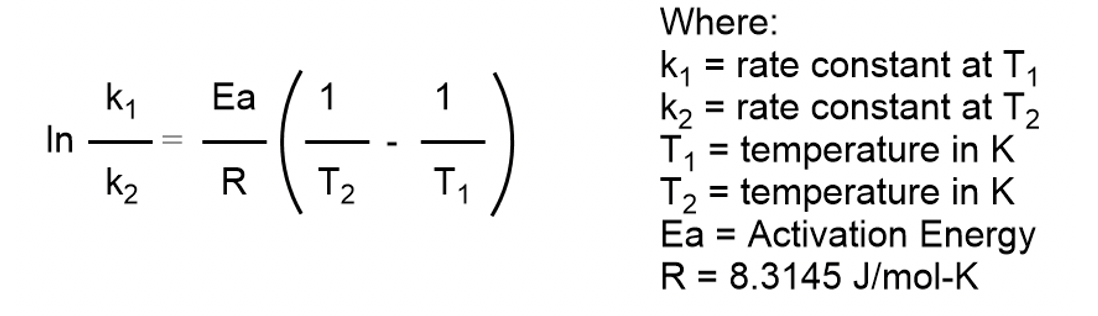Calculate the activation energy, E a Ea , in kilojoules per mole for a reaction at 57.0 ∘ C 57.0 ∘C that - brainly.com
The rate constant of a reaction is 1.2×10^ 3sec^ 1 at 30℃and 2.1×10^ 3sec^ 1 at 40℃.calculate the energy of activation of the reaction

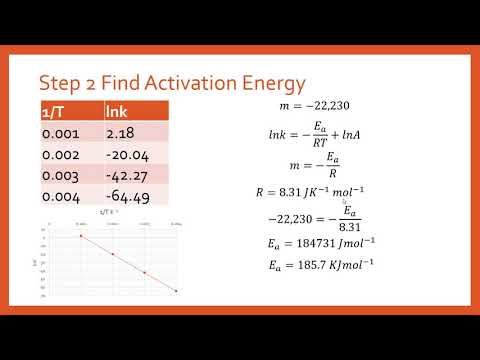
16.3.2 Determine activation energy (Ea) values from the Arrhenius equation by a graphical method. - YouTube

SOLVED: The activation energy Ea can be defined from the Arrhenius expression as: Ea = RT^2 / (2.303 * T^2 * bmax * Vrex) To show Ea = (1/2) kT + e*,

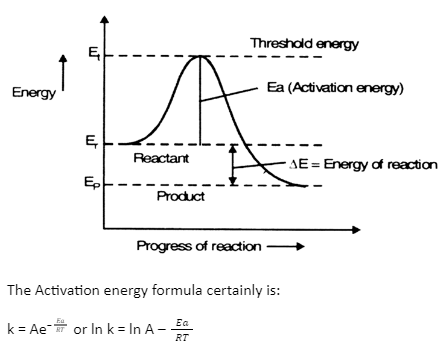
The activation energy the reaction, 2 HI(g) + H2 + 12(g) is 209.5kJmol-1 581 K. Calculate the fraction of molecules of reactants having energy equal to or greater than activation energy? SOLUTION