An inductively coupled plasma mass spectrometry method for the determination of elemental impurities in calcium carbonate mineral medicine - ScienceDirect

Identification and Characterization of an Isolated Impurity Fraction: Analysis of an Unknown Degradant Found in Quetiapine Fumarate | Waters

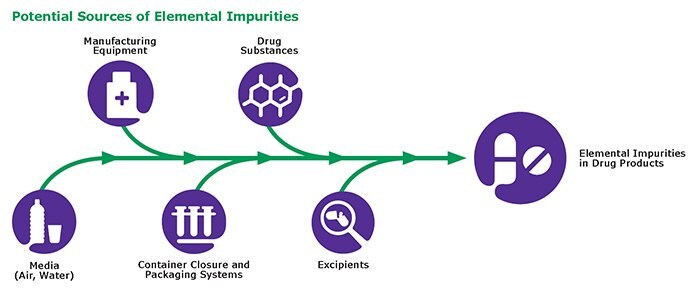
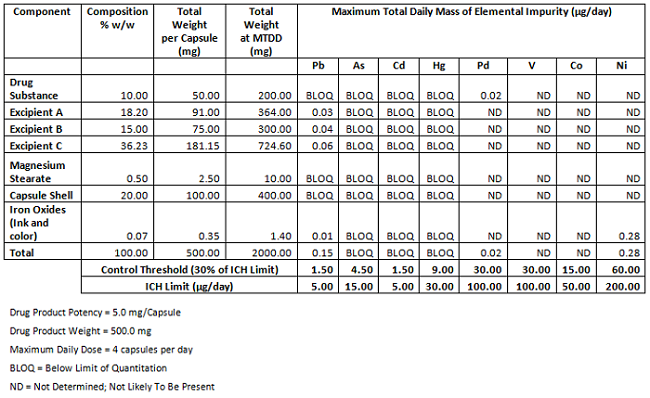
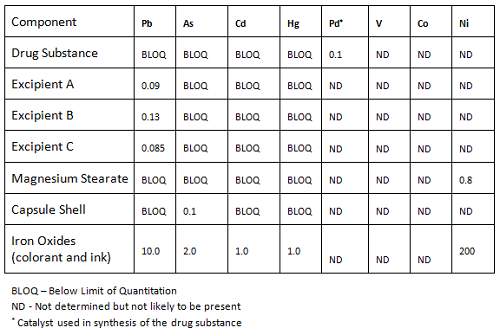
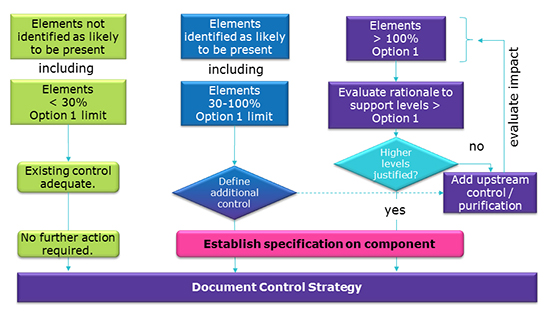
Elemental Impurities: Implications for Manufacturers of Drug Products, APIs, and Excipients | American Pharmaceutical Review - The Review of American Pharmaceutical Business & Technology
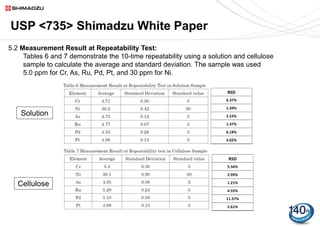
USP 735 as an Alternative to USP 233 for Elemental Impurity Analysis in Pharmaceutical Products | PPT