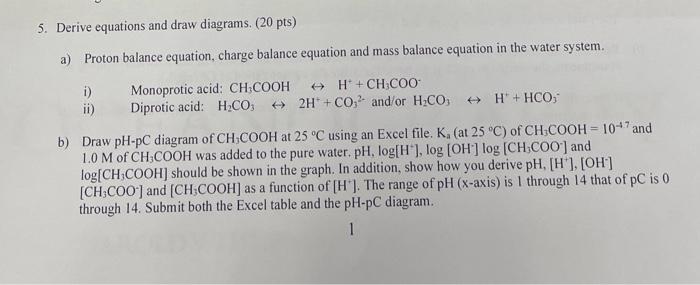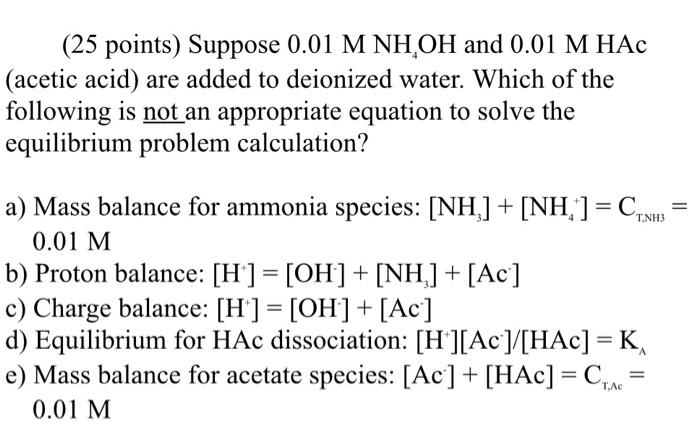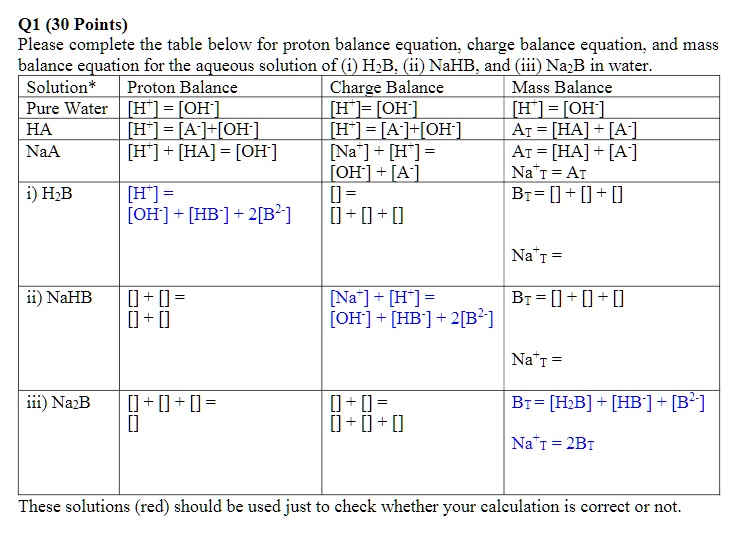
SOLVED: Q1 (30 Points) Please complete the table below for proton balance equation; charge balance equation; and mass balance equation for the aqueous solution of (1) HpB (ii) NaHB and (iii) Na2B
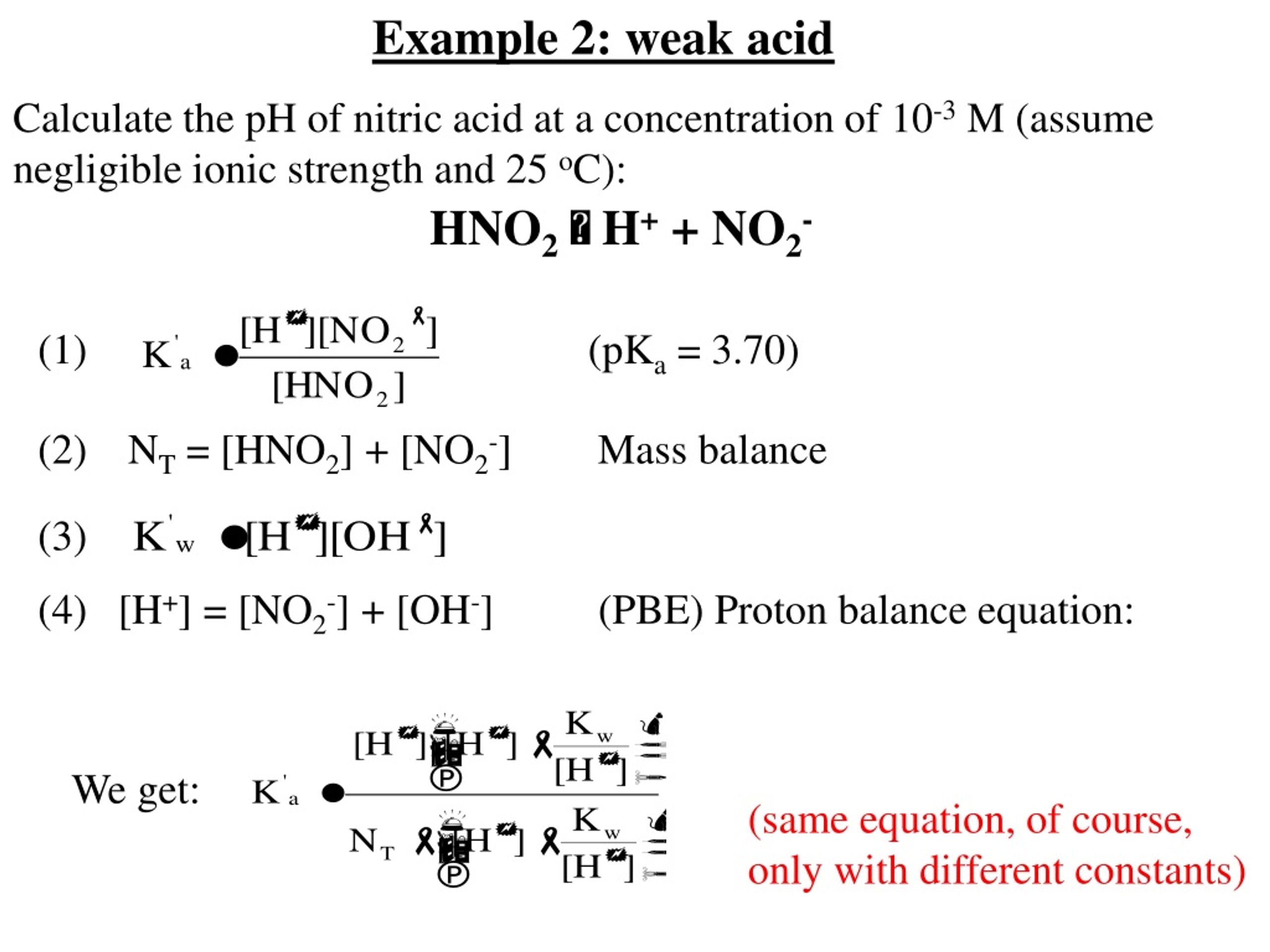
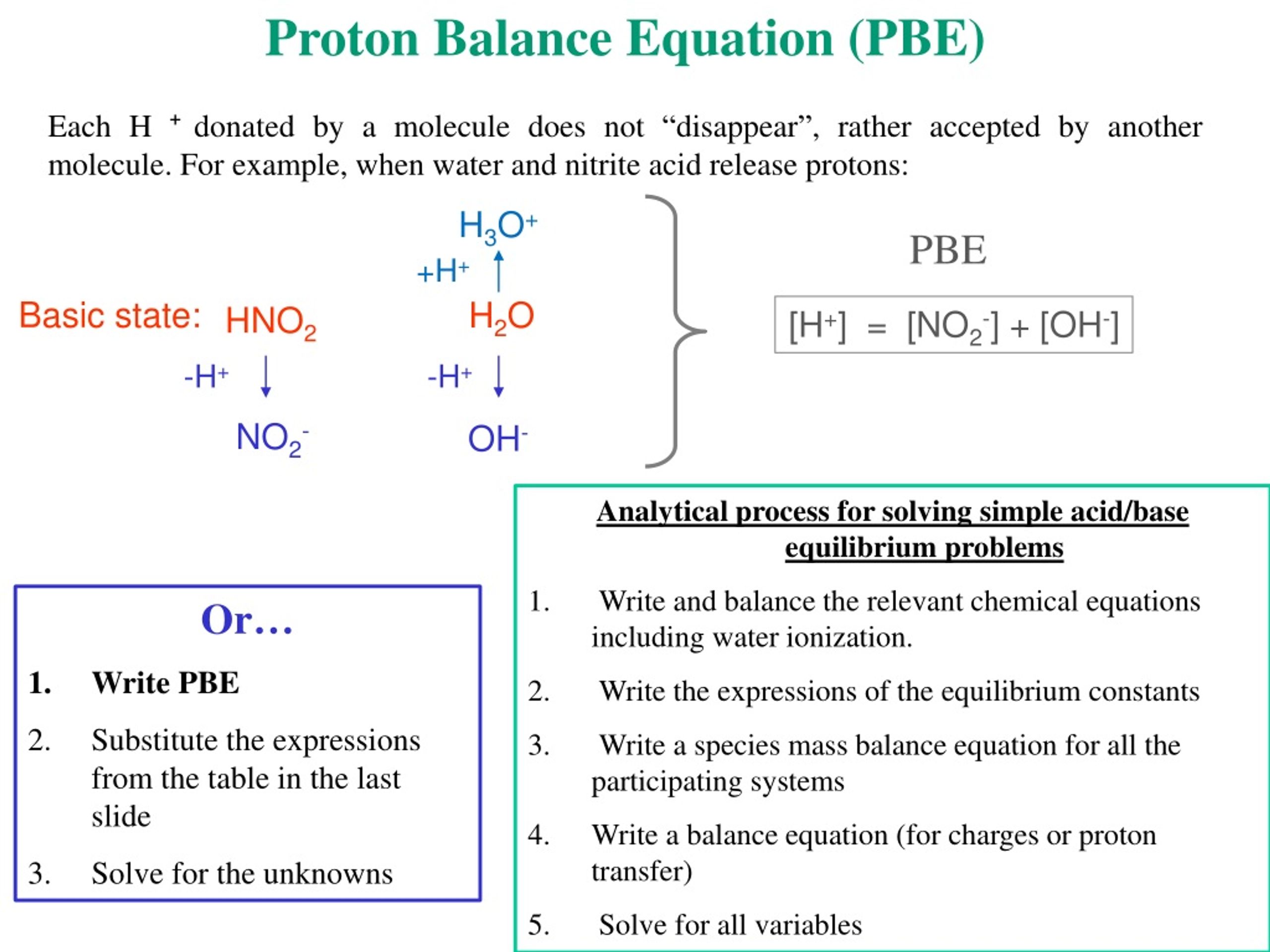
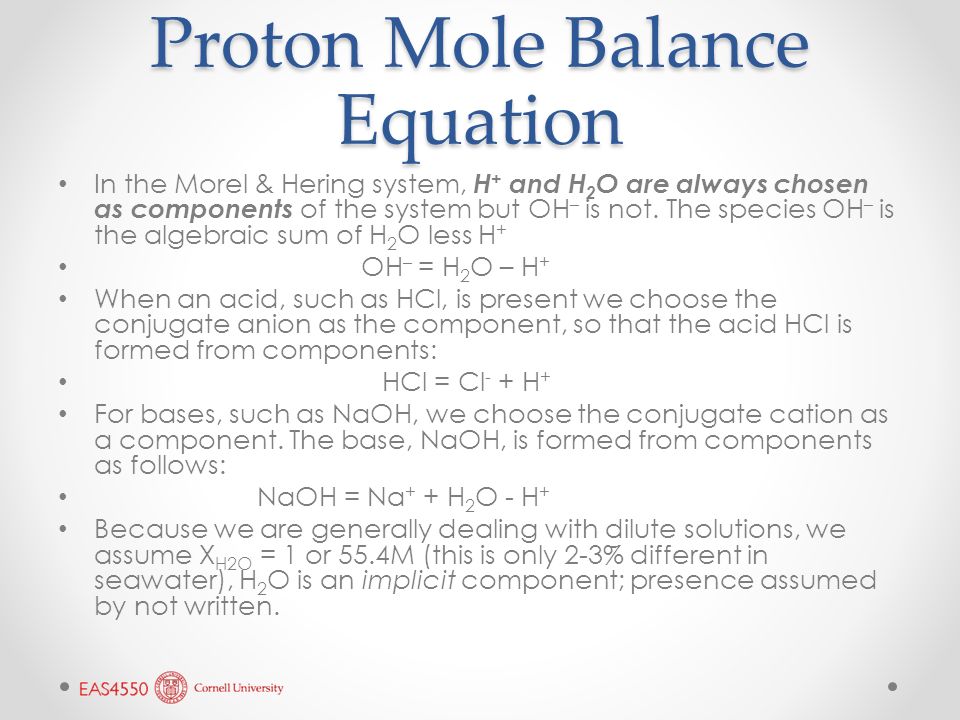
pH calculations and more in fundamentals of pharmaceutics. : Proton balance equation for weak monoprotic acids.
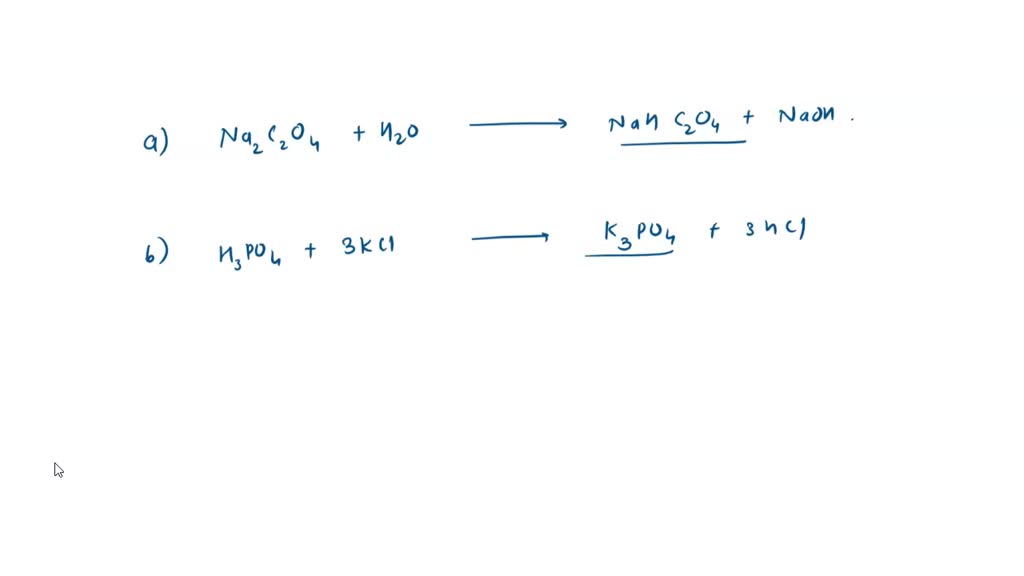
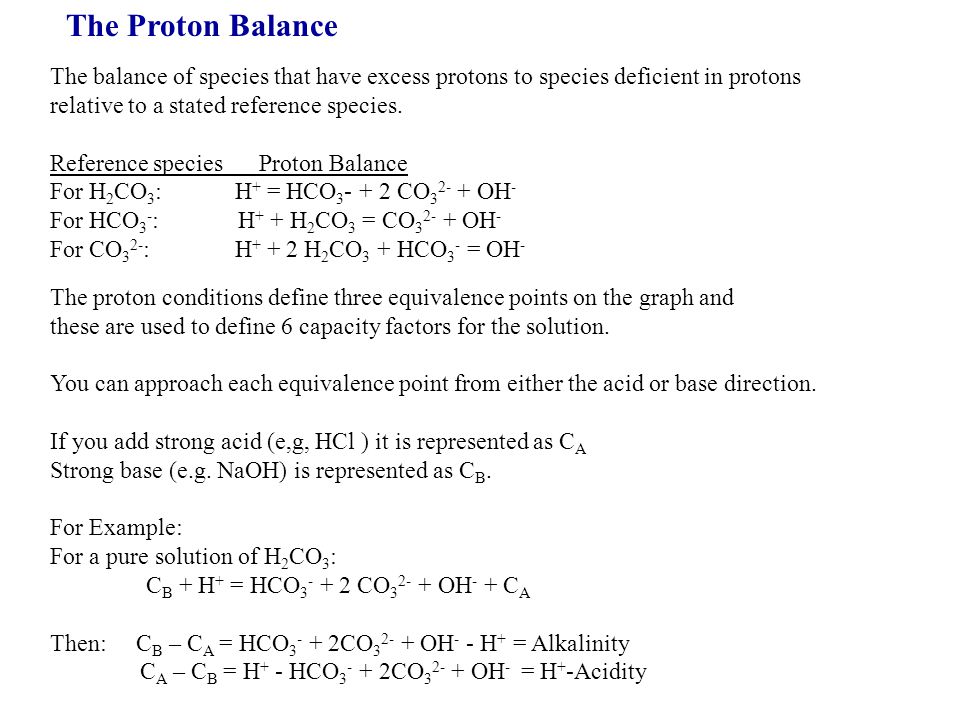
SOLVED: Write down the mass balance, charge balance, and proton balance equations for the following solutions: NaHCO3, K2PO4, NH4HCO3, NaHCO2, 0.1 M, and H2CO3, 0.1 M.

SOLVED: 2. Write the proton balance equations when the following salts are added to water: (a) NH4Acetate (b) NaHSO4 (c) Na2SO4 (d) Na3PO4 (e) NH4HCO3

Proton balance of the various types of N fertilizers: a plus indicates... | Download Scientific Diagram
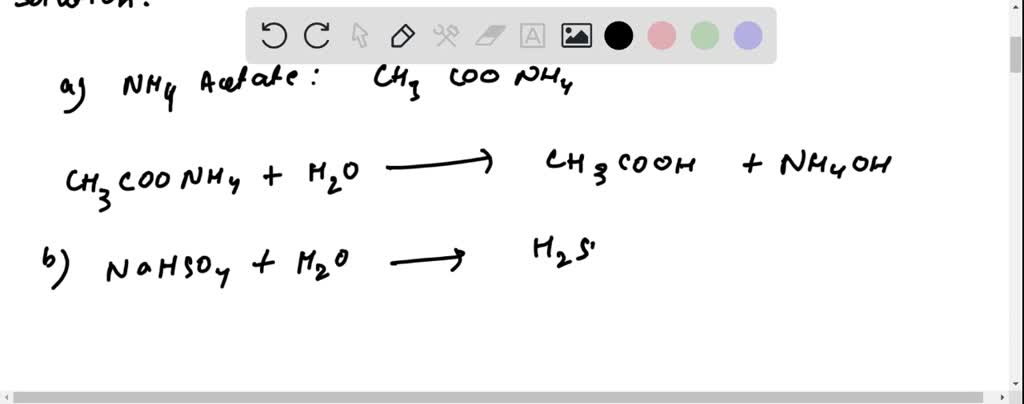
SOLVED: 2. Write the proton balance equations when the following salts are added to water: (a) NH4Acetate (b) NaHSO4 (c) Na2SO4 (d) Na3PO4 (e) NH4HCO3
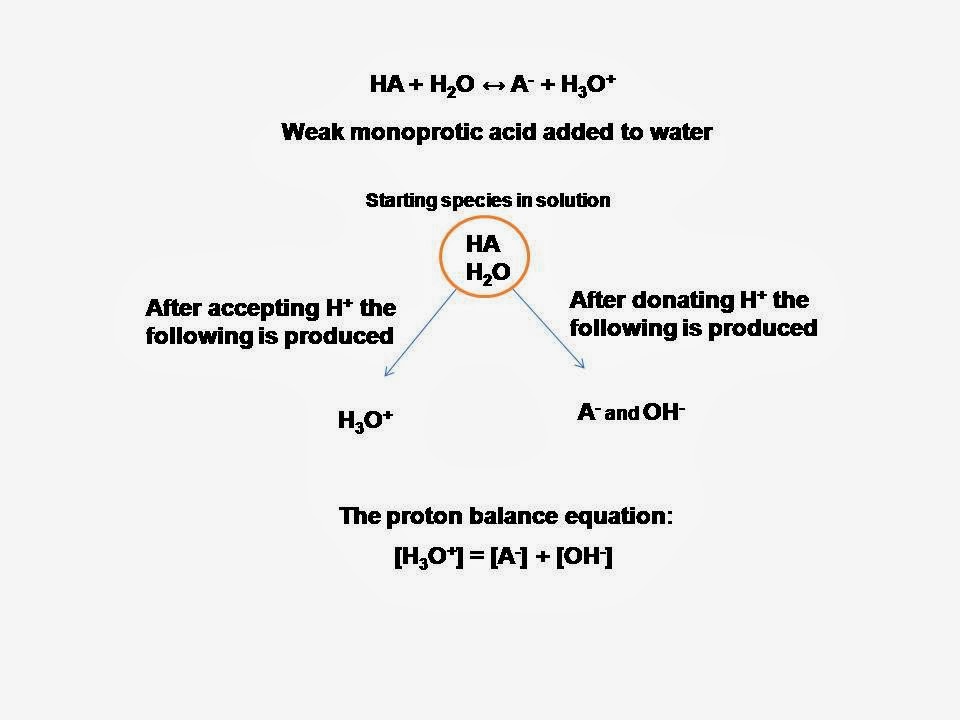
![SOLVED: The proton condition, with H2O and HAc being the reference species, is: [H+] - [OH-] + [Ac-]. In this case, the charge balance and proton condition are identical. The four equations ( SOLVED: The proton condition, with H2O and HAc being the reference species, is: [H+] - [OH-] + [Ac-]. In this case, the charge balance and proton condition are identical. The four equations (](https://cdn.numerade.com/ask_images/b28a2bc8f18d42c489078ab2a70bc9fa.jpg)
SOLVED: The proton condition, with H2O and HAc being the reference species, is: [H+] - [OH-] + [Ac-]. In this case, the charge balance and proton condition are identical. The four equations (

SOLVED: Write down the mass balance, charge balance, and proton balance equations for the following solutions: NaHCO3, K2PO4, NH4HCO3, NaHCO2, 0.1 M, and H2CO3, 0.1 M.